
A new indocyanine green fluorescence lymphography protocol for identification of the lymphatic drainage pathway for patients with breast cancer-related lymphoedema
Hiroo Suami1* , Asha Heydon-White1, Helen Mackie1,2, Sharon Czerniec1, Louise Koelmeyer1 and John Boyages1. BMC Cancer (2019) 19:985
Main findings
- Imaging procedures included:
- Phase one Observation of any spontaneous movement of ICG via the lymphatics for approximately 10min. Patients were encouraged to clench and unclench their hand ten times to facilitate lymphatic uptake of the ICG.
- Phase two Manual lymphatic drainage (MLD) massage was then performed by an accredited lymphoedema therapist to facilitate ICG transit via the lymphatics. They found that MLD facilitated dye movement more efficiently compared to post-injection exercise and delayed scanning although this was not formally evaluated. When lymphatic vessels were identified, their course was marked on the patient’s skin with a coloured pen.
- Phase three Demarcation lines of dermal backflow were marked on the skin, and collection of imaging data through still photography with both near infrared and digital cameras were taken.
- Lymphoedematous upper limbs were also classified by MDACC
- Stage as 0: normal lymphatics,
- Stage 1: many patent lymphatic vessels with minimal patchy dermal backflow,
- Stage 2: moderate number of patent lymphatic vessels with segmental dermal backflow,
- Stage 3: few patent lymphatic vessels with extensive dermal backflow involving the entire arm,
- Stage 4: no patent lymphatic vessels seen with dermal backflow involving the entire arm with extension to the dorsum of the hand and
- Stage 5: ICG does not move from injection sites
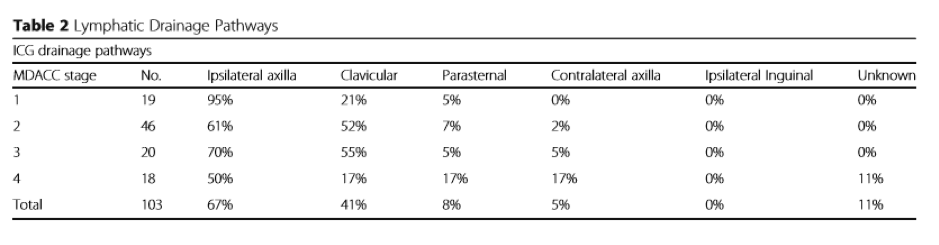
- Overall the percentage of drainage to the ipsilateral axilla was 67%. They found drainage to the ipsilateral axilla decreased as MDACC stage increased. As drainage to the axilla decreased by stage, they found drainage to the ipsilateral clavicular pathway increased reaching a peak of 55% for patients with MDACC Stage 3 lymphoedema.
- Patients with MDACC Stage 4 lymphoedema had the highest rate of drainage to the parasternal pathway and contralateral axilla (17%). In these cases, dermal backflow in the upper limb extended to the anterior midline of the chest and rerouted to the contralateral axilla via the intact lymphatic vessels in the contralateral breast. Of note, if there was a functional pathway to the proximal region of the ipsilateral upper limb, ICG did not extend beyond this region. For example, dermal backflow extended either to the parasternal region or to the contralateral axilla only when the pathway to the ipsilateral axilla or clavicular region was obstructed.
- The drainage pathway to the clavicular region was commonly identified for patients with MDACC ICG Stage 2 or 3 lymphoedema and occurred in 52-55% of patients respectively. It was apparent that sternal and contralateral pathway groups were found in Stage 4 lymphoedema.
- In recent, stress lymphoscintigraphy including exercise was introduced to improve lymphatic visualization but radiation exposure prevents applying MLD for facilitating tracer transit. In comparison ICG mixture moves faster than the lymphoscintigraphy tracer and facilitation of ICG transit with MLD can reduce examination time to specify the lymphatic drainage pathway and provides additional direct therapeutic guidance to the patient and the therapist.
- Another advantage of ICG lymphography is that some patients may indeed not suffer from lymphoedema. In this study four limbs in four patients were diagnosed as not having BCRL as they had normal lymphatic drainage without any dermal backflow. Future research should address the correlation of ICG lymphography with subclinical lymphedema detected by bioimpedance spectroscopy.
- The ipsilateral axilla is still considered a vital pathway. Axillary pathway was restricted functionally instead of complete obstruction in over two-thirds of patients.
Lower-limb oedema at the end of life: how common is it?
Megan Best, Edite Tang, Mark Buhagiar and Meera Agar. Journal of Lymphoedema, 2018, Vol 13, No 1
Main findings
- The International Lymphoedema Framework has proposed the term ‘oedema at the end of life’ (OATEOL) to cover all forms of oedema that develop as a result of multiple factors relating to terminal illness (International Lymphoedema Framework and Canadian Lymphedema Framework, 2010).
- It unlikely that pure lymphoedema exists in this population.
- Palliative care inpatients in two metropolitan palliative care units in Sydney, Australia. This was a cross-sectional, consecutive cohort study for the period of one calendar month (June 2013).
- All inpatients during the study period were screened for pitting lower-limb oedema through the normal process of admission to the unit. Pitting of the lower limb was assessed by applying sustained pressure for approximately 20 seconds against the medial malleolus and observing for persistent depression in the tissues after removal of pressure.
- Risk factor variables included medications at the time of admission that promote fluid retention, such as calcium blockers, NSAIDs, corticosteroids, or medications containing oestrogen; anticoagulant use and/or history of venous thromboembolic disease; cancer diagnosis; pelvic or abdominal involvement with tumour; past or current lower-limb cellulitis, ulcer or trauma; organ failure (renal, hepatic, cardiac); hypoalbuminaemia (serum albumin <32 g/L) if performed by the treating team within 3 days of admission; thyroid disease; and obesity. A global measure of functional status defined by Karnofsky (Australian) Performance Scale (AKPS) (Abernethy et al, 2005) was also collected to define level of immobility.
- 30 out of 59 had lower limb oedema.
- The most common risk factors were: cancer diagnosis (83%), medications that promote leg swelling through fluid retention (70%) and AKPS score <40 (63%).
- The prevalence of oedema is considerably higher than that of chronic oedema in the general community.
- The average number of risk factors per patient was 3.4. While some of these risk factors are unavoidable, such as diagnosis and disease distribution, others could conceivably be avoided. In view of the prevalence of OATEOL, more attention needs to be given to use of medications known to promote fluid retention in advanced disease. Similarly, this study supports initiatives to encourage exercise and weight control even in patients with life-threatening disease.
- Strengths of this study include a consecutive cohort, which reduces bias of the prevalence rate, and formal identification of the presence of oedema by a trained physiotherapist/medical officer. Limitations of this study included the small sample size and the age of the data.

