
Cancer- and Chemotherapy-Induced Musculoskeletal Degradation
Kathleen M Sturgeon, Katlynn M Mathis, Connie J Rogers, Kathryn H Schmitz,and David L Waning. JBMR Plus. 2019 Mar; 3(3): e10187
Click to read the abstract
Cancer- and Chemotherapy-Induced Musculoskeletal Degradation
Kathleen M Sturgeon, Katlynn M Mathis, Connie J Rogers, Kathryn H Schmitz, and David L Waning. JBMR Plus. 2019 Mar; 3(3): e10187
Mobility in advanced cancer patients is a major health care concern and is often lost in advanced metastatic cancers. Erosion of mobility is a major component in determining quality of life but also starts a process of loss of muscle and bone mass that further devastates patients. In addition, treatment options become limited in these advanced cancer patients. Loss of bone and muscle occurs concomitantly. Advanced cancers that are metastatic to bone often lead to bone loss (osteolytic lesions) but may also lead to abnormal deposition of new bone (osteoblastic lesions). However, in both cases there is a disruption to normal bone remodelling and radiologic evidence of bone loss. Many antitumor therapies can also lead to loss of bone in cancer survivors. Bone loss releases cytokines (TGFb) stored in the mineralized matrix that can act on skeletal muscle and lead to weakness. Likewise, loss of skeletal muscle mass leads to reduced bone mass and quality via mechanical and endocrine signals. Collectively these interactions are termed bone-muscle cross-talk, which has garnered much attention recently as a prime target for musculoskeletal health. Pharmacological approaches as well as nutrition and exercise can improve muscle and bone but have fallen short in the context of advanced cancers and cachexia. This review highlights our current knowledge of these intervention sand discusses the difficulties in treating severe musculoskeletal deficits with the emphasis on improving not only bone mass and muscle size but also functional outcomes.
Main findings
- Muscle weakness in patients with advanced cancer is associated with poor outcomes and exists as a spectrum that ranges from weakness in the absence of weight loss to profound muscle wasting and cachexia. Muscle weakness and loss of muscle mass affect between 15% and 80% of patients with cancer, depending upon tumour type and stage.
- A fivefold increase in fractures per year has been shown for women with newly diagnosed breast cancer receiving chemotherapy.
- Performance assessments of muscle function in cancer patients who received chemotherapy show slower chair-rise time, reduced hand-grip strength, and a decline in 12-minute walk distance compared with healthy control individuals.
- Doxorubicin and carboplatin chemotherapies have been used to study musculoskeletal changes and have revealed that these agents alone cause a significant reduction in bone volume.
- Increased risk of fracture arises from low bone mass, low bone strength, micro architectural disruption, and increased skeletal fragility. Further, fragility fractures (fractures that occur without trauma) are commonly found in the spine (vertebral compression fractures), hip, and wrist.
- Due to cancer therapy, cancer patients and survivors suffer from accelerated bone loss. Indeed, rates of bone loss from cancer therapy can be 10 times higher than in the general population.
- The onset of bone loss from premature menopause is sudden (6 months of treatment) and significant (21% decreased bone density compared with age-matched eumenorrheic women).
- A recent meta-analysis examined 21 independent studies in women aged 65 and younger, with stage 1 to 3 breast cancer, treated with tamoxifen or AIs, and showed:1)fracture risk was not elevated by tamoxifen use; 2) Aromatase Inhibitors (AIs) increased fracture risk by 17% compared with women who did not receive AIs; and 3) AIs increased fracture risk by 35% compared with women on tamoxifen. It was also observed that women on AIs have higher fracture risk during treatment than after treatment ends.
- Significant bone loss can occur in men with prostate cancer who are treated with ADT due to hypogonadism.
- Loss of bone mineral density can be detected after 6 to 9 months of ADT, and longer therapy confers a higher risk.
- The clinical definitions of sarcopenia include the presence of low skeletal muscle mass and either 1) low muscle strength, 2) low muscle function, or 3) low muscle performance.
- Cachexia is weight loss of at least 5% or more in 12 months or less in the presence of underlying illness, plus three of the following criteria: decreased muscle strength, fatigue, anorexia, low fat-free mass index, abnormal biochemistry (increased inflammatory markers [C-reactive protein >5.0mg/L,IL-6 >4.0pg/mL], anemia[<12g/dL],and low serum albumin [<3.5g/dL]).
- Within the setting of cancer, prevalence of sarcopenia has been reported to be 16% among long-term breast cancer survivors.
- The prevalence of cachexia varies by stage of cancer: 0.5% in all cancer patients versus 36% to 80% among advanced cancer patients.
- Itis difficult to assess the impact of chemotherapy directly on skeletal muscle in patients, but one study suggests that patients treated with neoadjuvant chemotherapy experience significant muscle wasting. In addition to loss of muscle mass, muscle weakness is an equally important adverse effect of cancer and cancer therapy (chemotherapy, radiation, and hormone deprivation) in cancer patients. This aspect is gaining attention as clinicians are beginning to directly assess physical activity and functional status in their patients.
- Exercise is recommended for maintenance of bone and muscle in patients undergoing treatment for cancer. Moderate intensity weight-bearing aerobic and resistance exercise is recommended to preserve and improve bone density in adult populations with and without cancer.
- Erosion of musculoskeletal function severely impacts the quality of life. In cancer patients, the loss of mobility and risk for falls and fractures are a major concern and can be caused by the tumour itself but also by the therapies used to reduce tumour burden. The loss of muscle mass and function (cachexia) and the loss of bone mass are connected in a feedback loop due to the tight interconnected mechanical and endocrine functions of these tissues.
- Most treatment strategies do not fully incorporate the protection of the musculoskeletal system even though this is critical to the quality of life and survival (Fig. 1). A key future direction is the incorporation of musculoskeletal protection and improved function early in the cancer continuum.
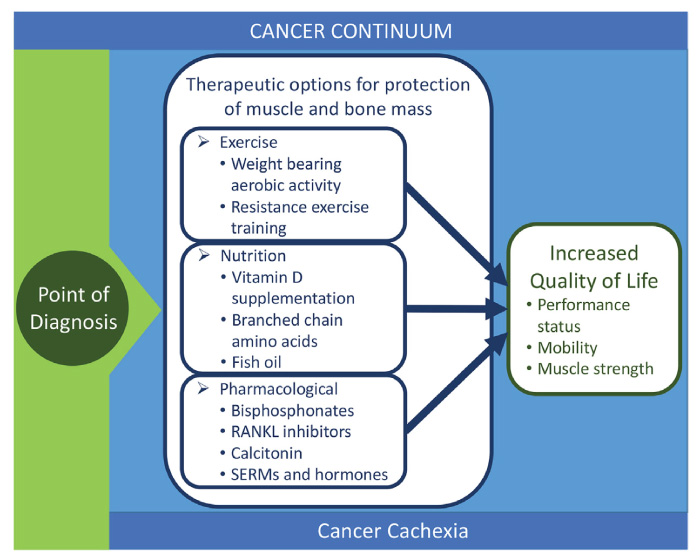
Fig. 1 – Schematic showing the therapeutic options for loss of bone and muscle throughout the cancer continuum.

