
Lymphedema following cancer therapy: overview and options
Michael Bernas, Saskia R. J. Thiadens, Betty Smoot, Jane M. Armer, Paula Stewart, Jay Granzow. Clinical & Experimental Metastasis, May 2018
Click to read the abstract
Lymphedema following cancer therapy: overview and options
Michael Bernas, Saskia R. J. Thiadens, Betty Smoot, Jane M. Armer, Paula Stewart, Jay Granzow. Clinical & Experimental Metastasis, May 2018
This summit focusing on lymphedema following cancer therapy was held during the 7th International Symposium on Cancer Metastasis through the Lymphovascular System. It was unique for the inclusion of patients with lymphedema joining physicians, therapists, healthcare professionals, and researchers to highlight what is known and more importantly what is unknown about the current state of research and treatment in the United States. The session opened with an introduction to lymphedema and then explored the incidence of multiple cancer-related lymphedemas, imaging tools and techniques useful for the diagnosis of lymphatic system abnormalities, and the new findings concerning the genetics of cancer-related lymphedema. It closed with a review of advocacy for patients and healthcare professionals and both conservative and surgical treatment options, followed by a panel discussion and questions. The session provided important information and updates which will be of value for improving the rehabilitation and overall support of patients with cancer-related lymphedema.
Main findings
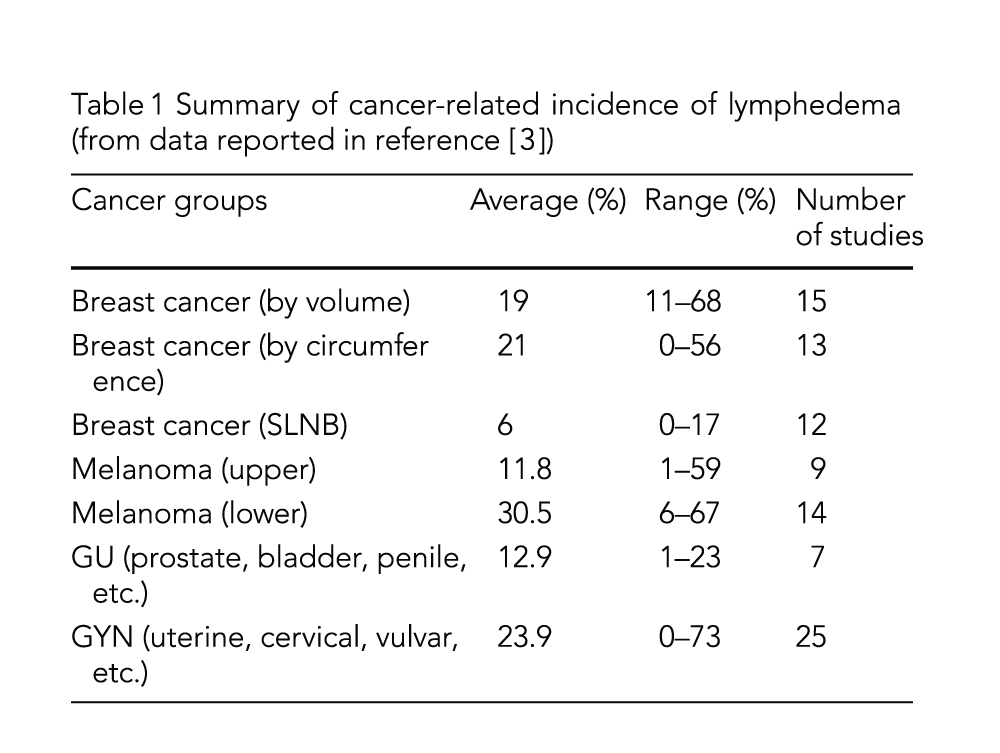
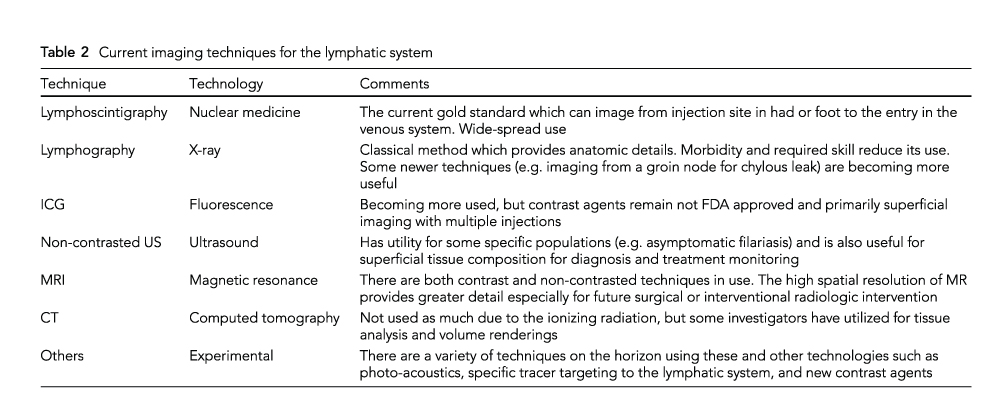
Genetics
- The variability seen in patients with breast cancer-related lymphedema, and described this variability as phenotypic heterogeneity. This phenotypic heterogeneity can be seen in the time to onset of lymphedema, progression of the condition, the amount of volume change, changes in the skin, the amount of inflammation, and a tendency toward fibrosis and adipose deposition.
- Phenotypic variability may also be manifest in variability in structure and function of the components of the lymphatic system. Recent research suggests that genomic variability and variability in molecular mechanisms may contribute to the phenotypic variability we can see, and in the phenotypic variability that we can’t see.
- There may be a genetic predisposition to secondary lymphedema, but more work is needed.
- Although the findings are preliminary and more, larger studies (which will allow further analysis of interaction effects) are needed, they did find statistically significant associations between specific single nucleotide polymorphisms (SNPs) and development of lymphedema after breast cancer treatment: (1) variations in 4 lymphatic and blood angiogenic genes and 3 haplotypes were associated with increased or decreased odds; (2) for cytokine genes, variations in 3 were associated with development of BCRL; and (3) six 6 SNPs were found across 4 potassium channel genes—4 SNPs were associated with having decreased odds of having lymphedema and 2 were associated with increased odds.
- These findings, once validated, may lead eventually to genetic screening for assessing risk for developing breast cancer-related lymphedema, but much more work and replication needs to be done.
Advocacy and treatment
- Although there is an understanding that prevention may be possible or at least lead to an inhibition of progression, this area still needs much more examination.
- There have been some interesting and positive findings using both surgical and non-surgical methods, but these studies are small and in selected populations.
- Industry has also been assisting by providing ever-improving devices for detection of small changes which may allow even earlier detection. Techniques such as bioimpedance spectroscopy and measuring devices such as the peromenter are becoming more widely used

